Human genome is the blueprint of life. The genome is highly complex with 3 billion nucleotides and 25,000 genes. Many human diseases are caused by defects in the structure or expression of the genes. Knowledge about the functions of individual genes and how they work together holds the keys to our understanding of the mysteries of life as well as strategies to disease treatment. It has always been scientists’ dream that someday, we will be able to precisely and effectively modify individual genes and “edit” out the defective genes. Because of the breakthrough discoveries of the recipients of this year’s Tang Prize in Biopharmaceutical Science, that “someday” is “today”.
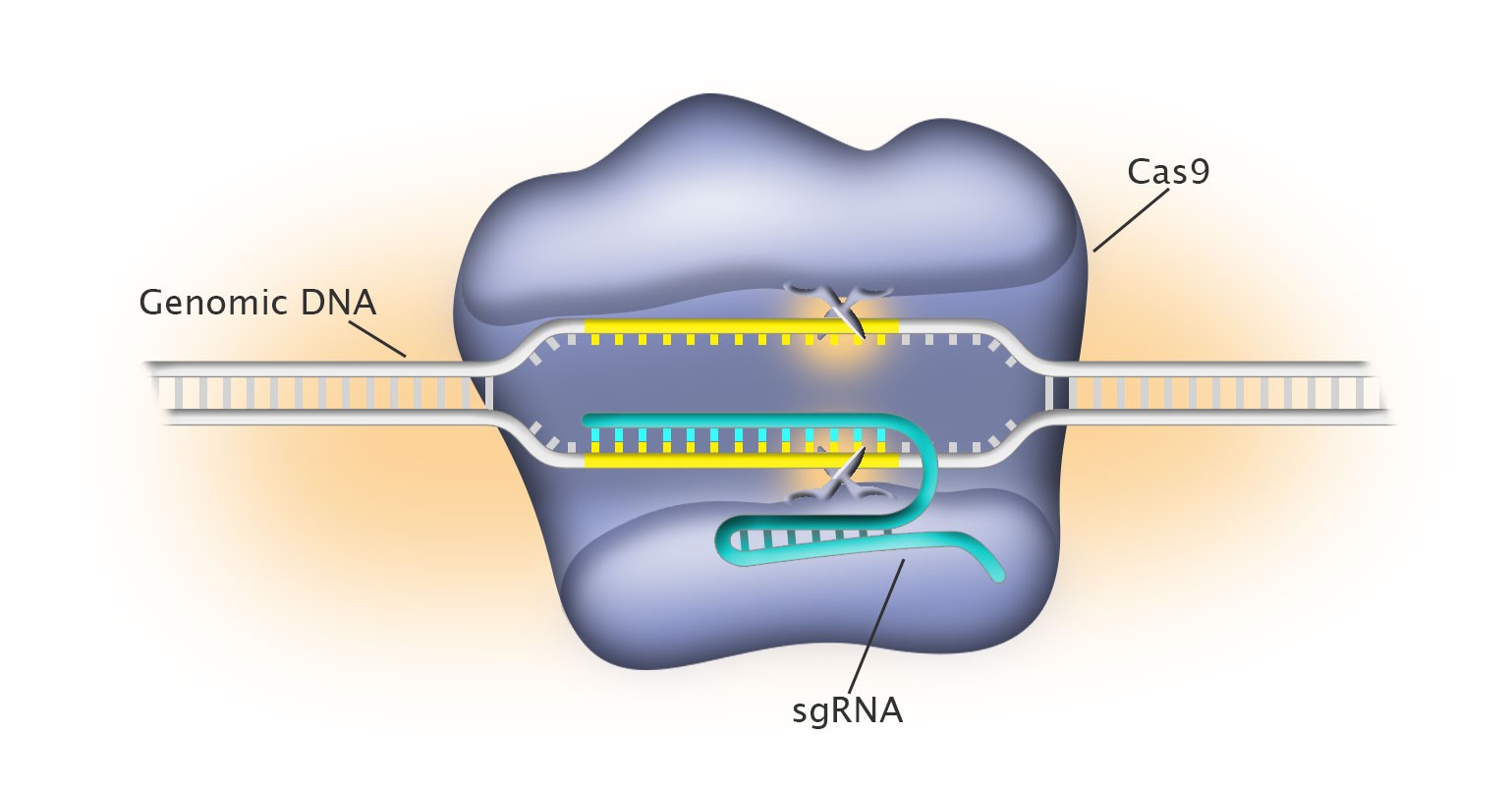
Considering the complexity of the genome, to edit individual genes at the nucleotide level is indeed similar to finding “a needle in the haystack”. Several genome editing approaches have been developed in the past with some success, but none as efficient, precise and sensitive as the CRISPR/Cas9 platform developed by Dr. Emmanuelle Charpentier, Dr. Jennifer Doudna and Dr. Feng Zhang. CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins) was originally identified in bacteria and archaea as their adaptive immune defensive systems against invading foreign DNA (such as in phage infection). Cas9 is an endonuclease which cuts DNA. In the past few years, there has been an explosion of interests in Cas9 to transform this prokaryotic nuclease into a versatile tool to engineer and edit eukaryotic genomes.
As an analogy, we can think of Cas9 as a pair of scissors which can precisely cut a string of pearls. The string of pearls is our DNA (or genome), which has 3 billion pearls or nucleotides. To pick out one particular pearl out of the 3 billion to cut is extremely difficult, if not impossible. Remarkably, Cas9 is equipped with a “GPS” system, in this case, RNA species, which can guide Cas9 to where the target DNA is. Dr. Charpentier first identified that there are two RNA species, the CRISPR RNA (crRNA) and the trans-activating crRNA (tracrRNA), which guide Cas9 to the target gene. Working with Dr. Charpentier, Dr. Doudna demonstrated that these two RNAs can be linked together to become a programmable single guide RNA (sgRNA) to target the gene of interest. The development of the two-component system (Cas9 and a single guide RNA) of Dr. Charpentier and Dr. Doudna have significantly simplified the experimental procedures and made efficient genome editing possible. Meanwhile, working independently, Dr. Zhang first reported the successful adaption of Cas9-based genome editing in mammalian and human cells. He further improved approaches for the simultaneous targeting of multiple genes and homology-based gene repair. The work of these three outstanding scientists have revolutionized the genome editing platform, making it programmable, accessible and scalable. Since their reports in 2012 and 2013, hundreds and likely thousands of labs world-wide have utilized this platform to engineer human cells and a variety of organisms including zebrafish, plants, monkeys, pigs and mice. Many believe that this is one of the greatest technology developments in the history of genome research, rivaling molecular cloning and PCR (polymerase-chain-reaction).
In addition to cutting and repairing genes, with some modifications, Cas9 can also be used to activate or suppress specific genes. The Cas9 system has already changed the way we do genetics and opened the floodgates for the discovery and development of new therapies that benefit human beings. Examples of the applications include the repair of defective genes in a large number of heritable diseases, such as sickle cell anemia, the fixing of mutated genes that drive cancer development, and the clearance of integrated genomes of cells chronically infected by viruses such as HIV (human immunodeficiency virus). The technology can be further adapted to engineer human stem cells for disease modeling, drug screening and identification of essential genes, tumor suppressors, regulators of cell differentiation, or genes sensitive to viral or pathogen infection. The potentials are enormous and limited only by imagination. Harnessing this revolutionary technology to improve various aspects of human health is already underway and huge impacts are anticipated.
Dr. Emmanuelle Charpentier, a French microbiologist, is well recognized as a world-leading expert in regulatory mechanisms underlying processes of infection and immunity in bacterial pathogens. She is presently Director of the Department of Regulation in Infection Biology, Max Planck Institute for Infection Biology. Her critical role in CRISPR is her original discovery that Cas9’s activity requires trans-activating crRNA (tracrRNA).
Dr. Jennifer Doudna is Professor of Chemistry and of Molecular and Cell Biology at the University of California, Berkeley and a Howard Hughes Medical Institute (HHMI) investigator. She is also a member of the National Academy of Science, USA. As a renowned RNA structural biologist, she has worked on ribozymes, IRES and more recently Cas9/RNA interactions. She, in collaboration with Dr. Charpentier, was credited for the discovery that crRNA and tracrRNA can be fused into a single guide RNA (sgRNA) for genome targeting.
Dr. Feng Zhang is an Investigator at the McGovern Institute for Brain Research, Core Member of the Broad Institute and Associate Professor of Brain and Cognitive Sciences and Biological Engineering at MIT. He was born in China, but moved to the United States when he was 11. He is a bioengineer with interests in developing tools for investigating neuroscience and human diseases. His previous work led to the development of “optogenetics” technology for regenerating nerve cells. His critical role in CRISPR technology was the first adaptation and demonstration in mammalian cells.



