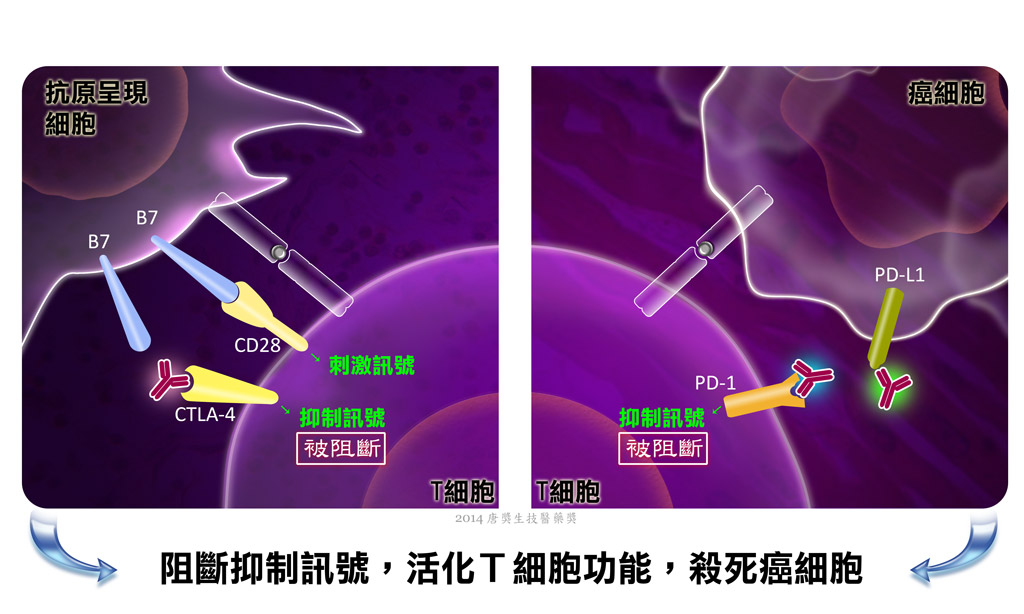
T細胞在免疫系統扮演重要角色,然而它仰賴抗原呈現細胞或其他分子,將外來或有害的抗原消化並且重新呈現成T細胞可以識別的抗原型態,T細胞才得以活化。此過程受到許多分子精密的調節。有的分子會促進T細胞活化,以確保免疫系統足以對抗入侵的病原體;有的分子則扮演抑制的功能,防止免疫系統過度活化。T細胞上有一群參與調節的蛋白質,被命名為CD28受體家族,因為CD28是此家族中首先被發現具有輔助受體功能的蛋白質。家族內的輔助受體分為兩大類,一類是負責傳遞刺激訊號的活化性受體,另一類是傳遞抑制訊號的抑制性受體。在抗原呈現細胞上,則存在其對應的配體分子,隸屬於B7家族。T細胞的抑制性受體當中,最受矚目的兩個受體是細胞毒性T淋巴細胞抗原4 (Cytotoxic T-Lymphocyte Antigen 4, CTLA-4) 和計畫性死亡-1 (Programmed cell death protein 1, PD-1)。而在抗原呈現細胞上,其對應的配體分別為 B7-1/B7-2 和 PD-L1/PD-L2。這些分子如今被稱為「免疫檢查點」受體和配體。
然而,精密複雜的免疫系統也有失靈的時候,或許也是很多疾病的基本成因。舉例來說,當抑制免疫的機能不正常減弱,反應過度的免疫系統將可能敵友不分,開始攻擊自身細胞組織,造成自體免疫性疾病。另外,免疫系統具有「免疫監控」機制,可以辨認並且消滅癌細胞。然而道高一尺,魔卻高了一丈,癌細胞也具備「免疫逃避」的機制。其中一招,是在自身癌細胞外表現B7家族配體,藉由和T細胞的抑制性受體作用,抑制T細胞的抗腫瘤作用。科學家企圖了解癌細胞逃避免疫監控的機制,針對這些路徑研發新的治療方法,使癌細胞無法抑制免疫反應,藉此強化免疫系統本身對抗癌症的能力。
詹姆斯•艾利森博士,目前為美國德州大學安德森癌症中心免疫系主任與免疫治療研究平台執行主任,是1995年發現CTLA-4是T細胞抑制性受體的二位科學家之一,他首先證明CTLA-4的抗體可以阻斷T細胞抑制性訊息,而達成活化T細胞殺死癌細胞的活性。他的團隊隨即製造出阻斷CTLA-4活性的抗體,並且於1996年以動物實驗證明該抗體可以排除小鼠體內數種腫瘤。而後經過多年努力,其單株抗體藥物成功被研發,經臨床試驗證明對末期轉移性黑色素瘤患者有顯著治療效果,且於2011年經美國食品藥物管理局 (FDA) 核准上市。
本庶 佑博士,目前為京都大學大學院醫學研究科免疫基因醫學講座之客員教授,於1992年發現PD-1。其研究團隊隨後證明PD-1是T細胞上的抑制受體。後來經其實驗室和其他實驗室證實,此蛋白質在腫瘤逃避機制上扮演著關鍵角色。這個發現引起眾多研究單位的興趣,競相發展此受體的阻斷劑,以達到治療癌症的效果。數種抗PD-1的抗體已由美國FDA核准為治療癌症的試驗用新藥。其中一株抗體經臨床試驗證實,對於非小細胞肺癌、轉移性黑色素瘤和腎細胞癌患者是完全有反應或是出現部分反應,且預期將於2015年核准上市,用以治療非小細胞肺癌;有專家預言PD-1與PD-L1阻斷劑深具潛力,將引領肺癌治療走向全新境界。另一株對非小細胞肺癌有顯著效果的抗體,目前也在進行多種癌症的臨床試驗。值得一提的是,使用抗CTLA-4抗體和抗PD-1抗體的組合來治療癌症,已經證實能顯著延長病患生命。
現在正逢癌症治療史上最令人興奮的時期。艾利森博士和本庶博士的發現,促使大家在免疫治療法上尋求新的契機,同時也讓許多難以治療的癌症曙光乍現。免疫檢查點異常也可能和癌症以外的疾病息息相關,例如過敏、傳染病和自體免疫性疾病。因此,著眼於免疫活化及抑制因子的研究,也將為上述疾病找到新的治療方向。
詹姆斯•艾利森博士與本庶 佑博士的發現帶領我們進入了醫藥新紀元。
學歷
|
1973
|
The University of Texas, Austin, TX, PhD, Biological Science
|
|
1969
|
The University of Texas, Austin, TX, BS, Microbiology
|
主要經歷
|
2012-Present
|
Chairman and Professor, Department of Immunology, The University of Texas MD Anderson Cancer Center, Houston, TX
|
|
2012-Present
|
Director, Immunotherapy Platform, The University of Texas MD Anderson Cancer Center, Houston, TX
|
|
2012-Present
|
Deputy Director, David H. Koch Center for Applied Research of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center, Houston, TX
|
|
2006-2012
|
Director, Ludwig Center for Cancer Immunotherapy, Memorial Sloan-Kettering Cancer Center, NY
|
|
2004-2012
|
Chairman, Immunology Program, Memorial Sloan-Kettering Cancer Center, New York, NY
|
|
2004-2012
|
David H. Koch Chair in Immunologic Studies, Memorial Sloan-Kettering Cancer Center, New York, NY
|
|
2004-2012
|
Attending Immunologist, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY
|
|
1997-2012
|
Investigator, Howard Hughes Medical Institute
|
|
1989-1997
|
Head, Division of Immunology, Department of Molecular and Cell Biology, University of California, Berkeley, CA
|
|
1987-1989
|
Interim Head, Division of Immunology, Department of Molecular and Cell Biology, University of California, Berkeley, CA
|
|
1985-2004
|
Professor, Division of Immunology, Department of Molecular and Cell Biology, University of California, Berkeley, CA
|
|
1985-2004
|
Director, Cancer Research Laboratory, University of California, Berkeley, CA
|
|
1983-1984
|
Visiting Scholar, Department of Pathology, Stanford University School of Medicine, Stanford, CA
|
|
1983-1984
|
Associate Biochemist and Associate Professor of Biochemistry, The University of Texas System Cancer Center, Science Park - Research Division, Smithville, TX
|
|
1977-1983
|
Assistant Biochemist and Assistant Professor of Biochemistry, The University of Texas System Cancer Center, Science Park - Research Division, Smithville, TX
|
|
1974-1977
|
Postdoctoral Fellow, Department of Molecular Immunology, Scripps Clinic and Research Foundation, La Jolla, CA
|
|
|
學術研究獎
| 2018 |
Nobel Prize in Physiology or Medicine (諾貝爾生理學或醫學獎) |
|
2014
|
Canada Gairdner International Award
|
|
2014
|
Szent-Györgyi Prize for Progress in Cancer Research
|
|
2014
|
Breakthrough Prize in Life Sciences
|
|
2013
|
AACR-CRI Lloyd J. Old Award in Cancer Immunology
|
|
2013
|
Economist’s Innovations Award for Bioscience
|
|
2011
|
Advancement of Cancer Research Award, Gilda’s Club
|
|
2011
|
Jacob Heskel Gabbay Award in Biotechnology and Medicine, Brandeis University
|
|
2011
|
Breakthrough Achievement in Translational Cancer Research, American Skin Association
|
|
2011
|
Roche Award for Cancer Immunology and Immunotherapy
|
|
2011
|
Lifetime Achievement Award, American Association of Immunologists
|
|
2010
|
Richard V. Smalley, MD, Memorial Lectureship Award, International Society for Biological Therapy of Cancer
|
|
2008
|
Dana Foundation Award in Human Immunology Research, American Association of Immunologists
|
|
2008
|
C. Chester Stock Award, Memorial Sloan-Kettering Cancer Center
|
|
2007
|
Elected Member, Institute of Medicine
|
|
2007
|
Fellow, American Association for the Advancement of Science
|
|
2005
|
William B. Coley Award for Distinguished Research in Basic and Tumor Immunology, Cancer Research Institute
|
|
2002
|
Outstanding Alumnus Award, The University of Texas at Austin Graduate School
|
|
2001
|
Centeon Award for Innovative Breakthroughs in Immunology
|
|
1997
|
Investigator, Howard Hughes Medical Institute
|
|
1997
|
Fellow, American Academy of Microbiology
|
|
1997
|
Member of the U.S. National Academy of Sciences
|
|
1997
|
Merit Award, National Institutes of Health
|
Harding F.A., McArthur J.G., Gross J.A., Raulet D.H., Allison J.P. CD28 mediated signaling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356: 607-009 (1992)
M., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182:459-465 (1995)
Leach D.R., Krummel M.F., Allison J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 271:1734-1736 (1998)
Van Elsas A., Hurwitz A.A., Allison J.P. Combination immunotherapy of 816 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTlA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF}-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by auto-immune depigmentation. J Exp Med 190:355-366 (1999)
Quezada S.A., Peggs K.S., Curran M.A., Allison J.P. CTLA-4-blockade and GMCSF combination immunotherapy increases effector frequencies altering the Intra-tumor balance of effector and regulatory T cells. J Clin Invest 7:1935-45 (2006)


