(This article is the outcome of a project collaboration between the Tang Prize Foundation and The Investigator Taiwan.)
Chinese original by Wai Keong Lim
Reviewed by Yan-an Zhang
The 2018 Tang Prize in Biopharmaceutical Science was jointly awarded to Dr. Tony Hunter, Dr. Brian J. Druker and Dr. John Mendelsohn for their contributions to basic science and its clinical applications that have led to important breakthroughs in cancer treatment. EGFR is the term often mentioned in articles about targeted cancer therapy. But how does EGFR cause cancer and why inhibiting EGFR is important to treating cancer? In this report, we will consult a research paper published in the journal Cells[1]in 2017 to investigate the connection between EGFR and cancer.
EGFR stands for epidermal growth factor receptor. They are “cell-surface receptors…that bind to ligands on the outside surface of the cell”[2] and they play an essential role in stimulating cell proliferation. “EGFR is involved in a variety of cellular processes,” including cell “survival, motility and differentiation.” What has been baffling scientists for a long time is how EGFR activates its downstream signaling pathways. To answer this question, two ideas have been proposed, namely the ligand-induced dimerization model and the rotation model. The former refers to the theory that “EGFR is activated by the ligand-induced dimerization of the receptor monomer, which brings intracellular kinase domains into close proximity for trans-autophosphorylation to initiate downstream signaling cascades,” while the later suggests that “EGFR is present as a pre-formed, yet inactive, dimer prior to ligand binding.” According to the rotation model, when ligands bind to the extracellular domain of EGFR, “its transmembrane domains rotate or twist parallel to the plane of the cell membrane,” leading to the reorientation of its intracellular kinase domain “from a symmetric inactive configuration to an asymmetric active form.” Further studies also indicate that “the homodimeric EGFR molecules are inactive, since EGF binding induces the phosphorylation and internalization of the receptor dimer,” which renders the rotation model even more convincing.
So what is the connection between EGFR and cancer? Different EGFR malfunctions can lead to cancer growth, including “the enhanced production of ligands, overproduction of the EGFR protein, mutations leading to the constitutive activation of EGFR and deficiency of EGFR downregulation.” The focus in this article is on the overexpression of EGFR, as we attempt to “explain the mechanisms of the constitutive activation based on the ‘rotation model.’” EGFR overexpression has been observed in many types of human cancer, making it one of the main targets of many cancer therapies. However, “in the absence of a bound ligand,” EGFR can still be activated by the overexpression of EGFR gene, and the overproduction of EGFR has been implicated in the formation of tumors. To put it in another way, while EGFR can exist as a preformed, inactive dimer, overexpression of EGFR can also induce constitutive activation of EGFR and activation of downstream proteins.
On the other hand, “mutations in the EGFR gene” also “frequently occur in human cancers.” An interesting fact about these mutations is that some mutants lack an EGFR extracellular domain but can still be “constitutively phosphorylated” and some researchers believe the rotation model can provide us with an explanation. Because “EGFR mutants with deleted extracellular ligand-binding domains” suffer an in-frame deletion of exons that encode certain amino acid residues, the deletion effects a structural change that makes the mutants “likely to trans-autophosphorylate” and as a result facilitate the development of malignant cells. This discovery has allowed us to understand EFGR’s propensity for phosphorylation and how it can cause cancer.
It is clear why EGFR became the protein many biochemists chose as a key target when developing cancer therapy drugs. With the advancement of biomedicine and personalized medicine, we will be able to have a deeper knowledge of the role EGFR plays in carcinogenesis and thus have the reason to believe that more EGFR inhibitors that can better meet our needs will be developed in the future.
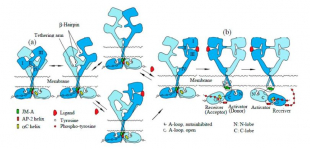
EGFR can exist as a preformed, inactive dimer. Ligand-binding to its extracellular domain will change the structure of its intracellular kinase domain to “an asymmetric active form,” which can drive the growth of cancer. Photo credit: https://doi.org/10.3390/cells6020013
[1] Please see the list of references at the end of this article. Unless otherwise stated, all the quotes in this translation are taken from “Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: the ‘Rotation Model’”
[2] “Ligands &Receptors.”
References:
1.Khan Academy. “Ligands & Receptors.” https://www.khanacademy.org/science/biology/cell-signaling/mechanisms-of-cell-signaling/a/signal-perception
2.Purba, E. R., Saita, E. I., & Maruyama, I. N. (2017). “Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: the ‘Rotation Model’”. Cells, 6(2), 13. https://doi.org/10.3390/cells6020013